On This Page
- All Heading 2s will automatically be pulled in to this list.
- Do not edit the content on this template.
Who are the partners on the NCI Cervical Cancer ‘Last Mile’ Initiative?
- Public sector partners include the NCI/NIH and the Food and Drug Administration (FDA). NCI interacts with other public sector agencies who may support the ‘Last Mile’ Initiative through initiatives such as the Federal Cervical Cancer Collaborative.
- Private sector partners include assay and device manufacturers seeking to obtain or expand regulatory approvals for self-collection approaches for primary HPV testing-based cervical cancer screening.
- Other partners include professional societies and academic and community groups interested in expanding access and implementing self-collection-based approaches.
What are the current key activities of the ‘Last Mile’ Initiative?
- Facilitating Regulatory Strategy for Self-Collection Approvals: Since 2019, in partnership with public and private sector stakeholders, the ‘Last Mile’ Initiative has conducted meta-research and facilitated stakeholder discussions to facilitate regulatory pathways. This work, in part, led to FDA’s approval decisions in May 2024 to expand the current label/indication of use for two approved tests (BD Onclarity and Roche cobas HPV). The FDA has permitted vaginal self-collected samples using specifically approved devices (Copan FloqSwab and Rovers Evalyn Brush) and specific instructions for use (IFU) information as acceptable alternative specimen types when cervical sampling by a healthcare provider is either contraindicated or cervical specimens otherwise cannot be obtained. This is the first step in a multi-level regulatory strategy that will subsequently consider additional accumulating evidence (such as the SHIP Trial, see below) to expand these approvals to include collection outside of a healthcare settings, including at-home use.
- SHIP Trial: In 2024, NCI launched the ‘Self-collection for HPV testing to Improve Cervical Cancer Prevention’ (SHIP) Trial, a multi-center study focused on evaluating the usability, acceptability, accuracy, and effectiveness of self-collection devices for primary HPV testing. This trial is being conducted as a partnership with HPV assay/device manufacturers, will contribute both pre-approval and post-approval data necessary for FDA’s regulatory actions on self-collection.
- Disseminating Evidence for Implementation: The ‘Last Mile’ Initiative will work with partners to develop approaches to disseminate evidence on self-collection approaches to update clinical practice guidelines and inform implementation strategies.
If self-collection is approved by the FDA, why is the SHIP Trial being done?
The current FDA-approved labels require vaginal self-collected samples to be obtained only using specifically approved devices in a clinical environment where patients have an opportunity to get assistance from healthcare providers and where samples can be handled and processed by trained personnel. The SHIP Trial is being done to expand these approvals further in three key ways:
- The SHIP Trial will generate new US-based data on self-collection done outside a healthcare setting (e.g., at-home) and without assistance by a healthcare provider leads to robust accuracy as compared to clinician-collected samples. In addition, the study will also generate evidence on patient-level factors (including usability, acceptability, and preferences) to inform adoption of self-collection from the intended use population that may benefit the most from self-collection as an alternative. To this end, NCI is working with assay manufacturers to conduct an independent evaluation of their ‘at-home self-collection kits’ in the SHIP Trial. This evidence will be submitted to the FDA so they can consider expansion of the current indications to include ‘at-home’ self-collection in the future.
- As part of a FDA-mandated post-approval requirement imposed in the May 2024 approval orders for the two assay manufacturers, the SHIP trial provides a neutral and independent platform for a non-competitive evaluation for a confirmatory trial on the clinical accuracy of HPV testing when done by the newly approved specific self-collection devices when used in healthcare settings.
- The NCI is also working with private sector partners who have developed novel self-collection devices and approaches for their neutral and independent evaluation in the SHIP Trial, such that the FDA can rely on this additional evidence when considering these newer devices as alternatives to clinician-collection when used with currently approved HPV testing assays.
How does the SHIP Trial relate to the ‘Last Mile’ Initiative?
The SHIP Trial is one of the key activities of the ‘Last Mile’ Initiative. The evidence generated in this study will contribute necessary data for advancing FDA regulatory actions on self-collection-based HPV screening approaches.
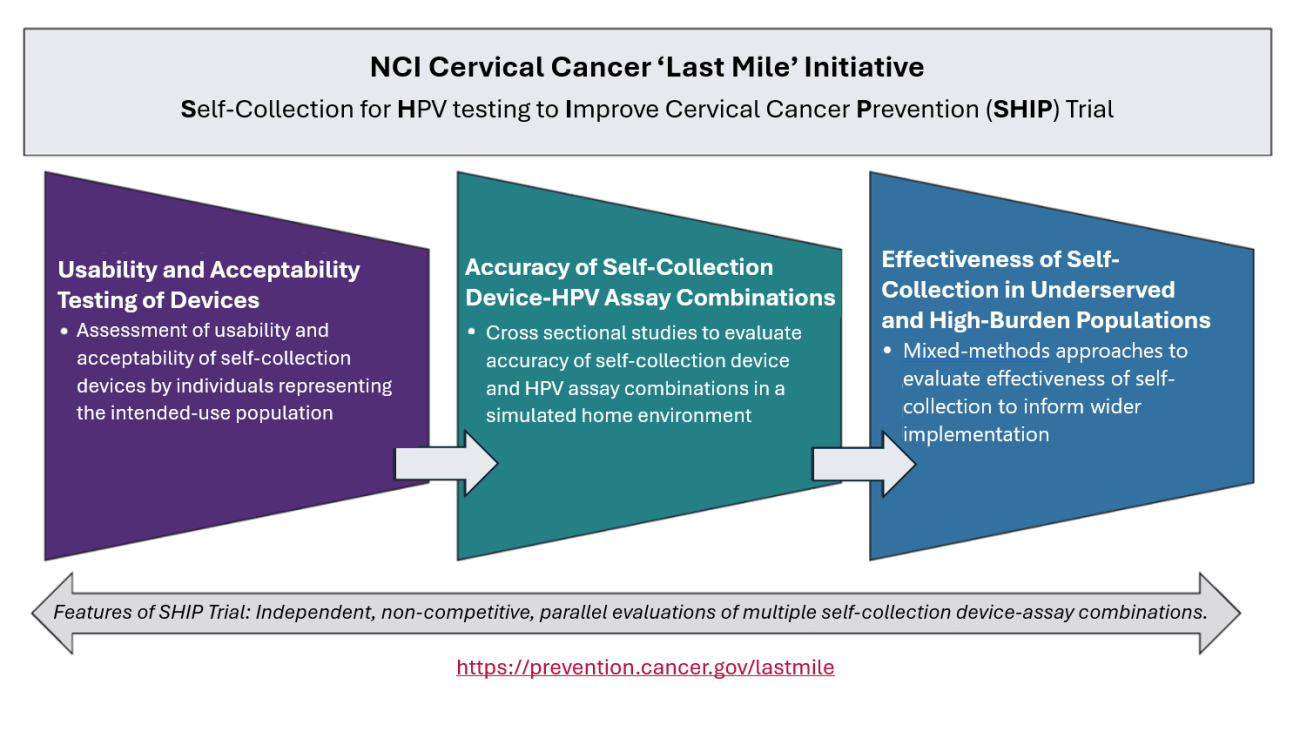
Self-collection for HPV testing to Improve Cervical Cancer Prevention (SHIP) Trial involves:
Usability and Acceptability Testing of Devices
- Assessment of usability and acceptability of self-collection devices by individuals representing the intended-use population
Accuracy of Self-collection Device-HPV Assay Combinations
- Cross sectional studies to evaluate accuracy of Self-collection device and HPV assay combinations in a simulated home environment Effectiveness of Self-collection in Underserved and High-Burden Populations
- Mixed-methods approaches to evaluate effectiveness of self-collection to inform wider implementation
Features of SHIP Trial: Independent, non-competitive, parallel evaluations of multiple self-collection device-assay combinations.
I am an investigator or clinician conducting cervical cancer screening. Can my clinic participate in the SHIP Trial?
At this time, the Clinical Enrollment Sites have been selected for the first phase of the study. If you are interested to be considered as a future site for the SHIP trial or other related research, please contact CervicalCancerLastMile@nih.gov for more information.
